Amid Variants 16-17 Year Olds are Able to Get Booster
Photo Credit: Reuters
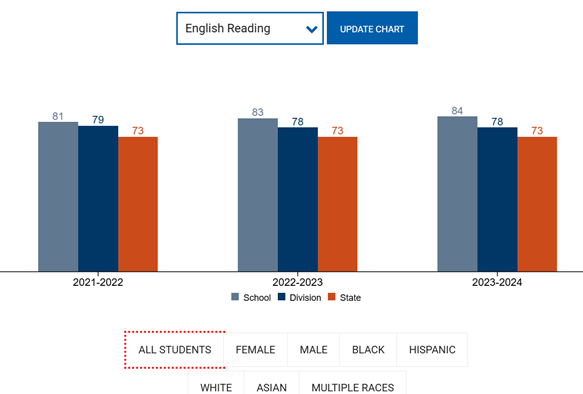
On Thursday December 9, 2021, The U.S. Food and Drug Administration authorized the Pfizer/BioNTech vaccine booster for 16-17 year olds. This age group is eligible to receive the booster shot six months after their second dose of the Pfizer/BioNTech vaccine. If an individual of 16 or 17 years of age has previously received the Moderna or Johnson & Johnson vaccine, they are not eligible to receive the Pfizer/BioNTech booster. Individuals 18 and older, however, are able to get any of the authorized COVID-19 vaccines.
According to a trial performed by Pfizer and BioNTech, the booster dose given to 16 year olds and older is more than 95% effective when compared with those who did not receive a booster. The booster dose has increased protection against COVID-19 and its variants significantly to almost the level of protection of the two-dose vaccine.
It is highly encouraged by medical professionals to get the booster because it keeps immunity up, since vaccinations become less effective over time. With the rise of more infectious and deadly variants, such as Delta and Omicron, the importance of this booster shot increases.
These advancements regarding the vaccine mark a significant step towards stopping the spread of COVID-19 and its variants. Medical professionals continue to urge people of all ages to get vaccinated, but they say there needs to be more information before making a decision about approving boosters for children under 16.
Here is a website to find a vaccine.
Adeline Sauer is a determined senior who brings diverse interests and a strong work ethic to journalism. She is one of the School and Student Issue Co-Editors...